
Introduction
Betibeglogene autotemcel (beti-cel; LentiGlobin for β-thalassemia) gene therapy is being evaluated for the treatment of transfusion-dependent β-thalassemia (TDT). Initial positive results of beti-cel in the phase 3 studies, HGB-207 (NCT02906202; non-β0/β0 genotypes) and HGB-212 (NCT03207009; β0/β0, β0/β+ IVS-I-110 and β+ IVS-I-110/β+ IVS-I-110 genotypes), showed 10/12 adult patients achieved transfusion independence. The studies expanded enrollment to include adolescents and children. We present interim results from pediatric patients <18 yrs who received beti-cel in HGB-207 and HGB-212 as of 3 March 2020.
Methods
After mobilization and apheresis, autologous CD34+ cells were transduced ex vivo with BB305 lentiviral vector, containing a modified human β-globin gene to produce beti-cel drug product (DP). Patients underwent busulfan myeloablation and infusion with beti-cel and were then followed longitudinally. Transfusion independence (TI; weighted average hemoglobin [Hb] ≥9 g/dL without transfusions for ≥12 mo) was the primary endpoint in HGB-207 and a secondary endpoint in HGB-212. Transfusion reduction (≥60% reduction in transfusion volume between Month 12 to 24 versus baseline) is the primary endpoint in HGB-212. Hb levels, TI characteristics, and quality of life were secondary endpoints. Assessments of ineffective erythropoiesis were exploratory. Data presented as median (min-max).
Results
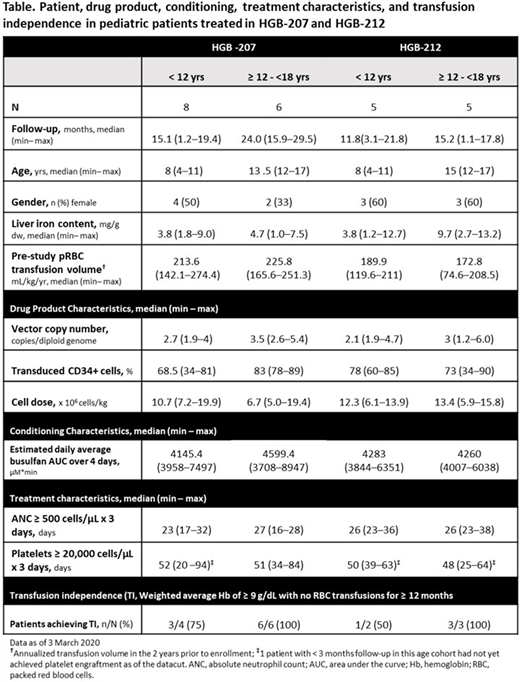
Twenty-four pediatric patients were treated including 13 patients <12 yrs old (207: n=8; 212: n=5) and 11 patients ≥12 to <18 yrs old (207: n=6; 212: n=5). Patient, DP, and engraftment characteristics are described in the Table. All patients achieved neutrophil engraftment. Twenty-one patients with >3 mo follow-up achieved platelet engraftment and had platelets ≥100 x109/L by Month 12; one 17-yr old patient did not have platelets ≥100 x109/L until Month 15.
In HGB-207, 6/7 (86%) patients <12 yrs and 6/6 (100%) patients ≥12 to <18 yrs with >3 mo follow-up have stopped transfusions for ≥6 mo. TI was achieved in 3/4 (75%) evaluable patients <12 yrs with weighted average Hb during TI of 10.0 (9.4-10.3) g/dL. At last visit, gene therapy-derived HbAT87Q in these patients was 5.1-7.1 g/dL. In patients ≥12 to <18 yrs, all 6 patients achieved TI with weighted average Hb during TI of 11.6 (11.3-12.3) g/dL. These patients had 8.4-10.5 g/dL HbAT87Q at last visit. Myeloid:erythroid (M:E) ratio in patients <12 yrs with TI improved from 1:3.7-1:1.1 at baseline to 1:2-1.5:1 at Month 12; in patients ≥12 to <18 yrs with TI, M:E ratio improved from 1:7.3-1.6:1 at baseline to 1:2.7-1.9:1 at Month 12. Patients <18 yrs who achieved TI in HGB-207 had an improved health state today score as assessed using EQ-5D-Y from 67 (50-96) at baseline (n=7) to 92.5 (85-95) at Month 12 (n=6).
In HGB-212, 3/5 (60%) patients <12 yrs and 4/4 (100%) patients ≥12 to <18 yrs with >3mo follow-up have stopped transfusions for ≥6 mo. TI was achieved in 1/2 evaluable patients <12 yrs. Weighted average Hb during TI was 10.3 g/dL and HbAT87Q was 9.2 g/dL at last visit in this patient. All 3 evaluable patients ≥12 to <18 yrs achieved TI with weighted average Hb of 9.6 (9.5-12.8) g/dL. HbAT87Q in these patients was 8.0-10.9 g/dL at last visit. The M:E ratio in the patient <12 yrs with TI improved from 1:4.7 at baseline to 1.2:1 at Month 12. In the 2 patients ≥12 to <18 yrs with baseline and Month 12 assessments, M:E ratios improved from 1:5.1 to 1:1.4 and 1:3.3 to 1:1.8.
Post-infusion non-hematologic grade ≥3 adverse events (AEs) in ≥3 patients aged <12 yrs in either study were febrile neutropenia (n=9), stomatitis (n=6), decreased appetite (n=4), increased alanine aminotransferase (n=3), and epistaxis (n=3); in patients ≥12 to <18 yrs, these were stomatitis (n=8), febrile neutropenia (n=3) and hypoxia (n=3). Grade 4 veno-occlusive disease occurred in 2 patients ≥12 to <18 yrs and one grade 2 event occurred in a patient <12 yrs; all cases were successfully treated with defibrotide. Neither replication-competent lentivirus, insertional oncogenesis, nor clonal dominance were observed.
Summary
Interim results in HGB-207 and HGB-212 show that after treatment with beti-cel, pediatric patients <18 yrs achieved transfusion independence with comparable rates as in adults, suggesting that beti-cel gene therapy represents an effective treatment option across ages. The safety profile of gene therapy with beti-cel was consistent with busulfan myeloablation.
Thompson:CRISPR/Vertex: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Baxalta: Research Funding; BMS: Consultancy, Research Funding; Biomarin: Research Funding; bluebird bio, Inc.: Consultancy, Research Funding. Kwiatkowski:Novartis: Research Funding; Agios: Consultancy; Sangamo: Research Funding; bluebird bio,Inc.: Consultancy, Research Funding; Apopharma: Research Funding; Imara: Consultancy; BMS: Consultancy; Terumo Co: Research Funding; Celgene: Consultancy. Porter:Vifor Pharmaceuticals: Honoraria; bluebird bio, Inc.: Consultancy, Honoraria; Agios Pharmaceuticals: Consultancy, Honoraria; La Jolla Pharmaceuticals: Honoraria; Silence Therapeutics: Honoraria; Protagonist Therapeutics: Honoraria; BMS: Consultancy, Honoraria. Kulozik:Novartis: Consultancy, Honoraria; bluebird bio, Inc.: Consultancy, Honoraria. Thrasher:Rocket Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Generation bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Equity ownership; Orchard Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Equity ownership; 4Bio Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees. Thuret:Novartis pharma: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials; bluebird bio, Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials; Apopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Investigator in clinical trials. Lal:Insight Magnetics: Research Funding; Celgene, BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Terumo Corporation: Research Funding; Agios Pharmaceuticals: Consultancy; Chiesi USA: Consultancy; La Jolla Pharmaceutical Company: Research Funding; bluebird bio, Inc.: Research Funding; Protagonist Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Guo:bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Liu:bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Colvin:bluebird bio, Inc.: Current Employment, Current equity holder in publicly-traded company. Walters:Veevo Biomedicine: Consultancy; AllCells, Inc: Consultancy; Editas: Consultancy. Locatelli:Jazz Pharmaceeutical: Speakers Bureau; Medac: Speakers Bureau; Miltenyi: Speakers Bureau; Bellicum Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal